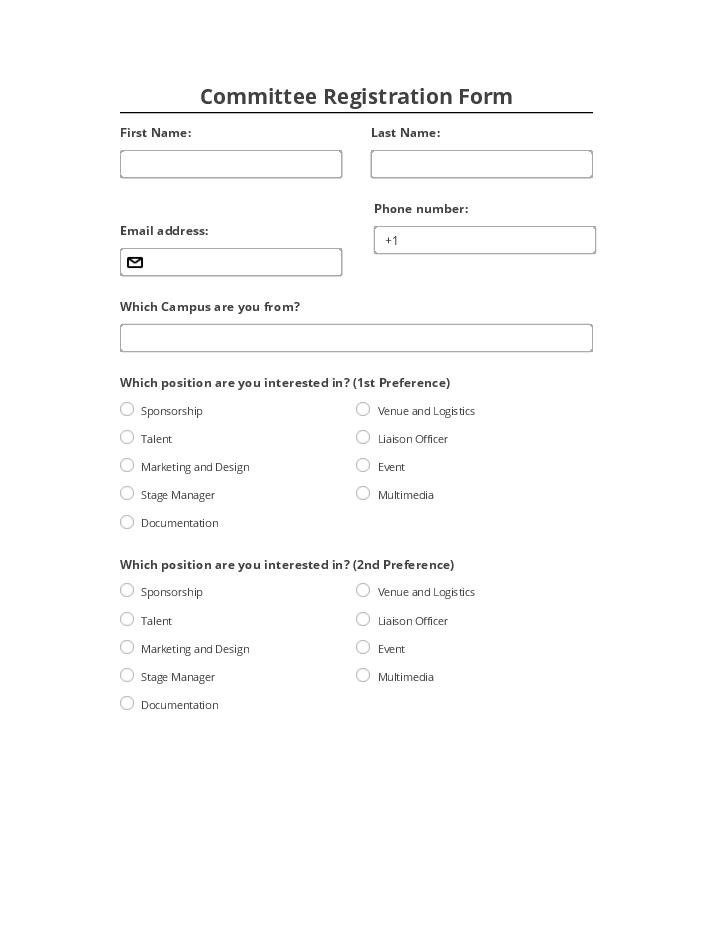
Pre-fill Committee Registration fields from a CRM, Spreadsheet or database records
-
Pre-fill from Airtable Bot Pre-fill documents with data from Airtable
-
Pre-fill dropdown from Airtable Bot Pre-fill dropdown fields with data from Airtable
-
Pre-fill with Custom Data Bot Pre-fill documents with custom values
-
Pre-fill from Doc to Doc Bot Pre-fill a document with data from another document or within the same document
Extract data from Committee Registration to a CRM, Spreadsheet or database
Archive Committee Registration to Google Drive, Dropbox, OneDrive and other cloud storage services
Assign recipients to fields and route the document automatically
Automate repetitive tasks in CRMs, G Suite, and other systems of record
-
Pre-fill from CSV file Bot Pre-fill documents with data from a CSV file
-
Pre-fill from CSV File dropdown options Bot Pre-fill dropdown fields with data from a CSV file
-
Pre-fill from Excel Spreadsheet Bot Pre-fill documents with data from a XLS/XLSX file
-
Pre-fill Dropdowns from Excel Spreadsheet Bot Pre-fill dropdown fields with data from a XLS/XLSX file
Save an average of 8 hours per week with an automated Committee Registration workflow
Spend an average of 10 minutes to complete a Committee Registration document